go through: Dr. Tanusri Chakraborty
As we move towards net zero and low carbon emissions, hydrogen (H₂) is expected to play a crucial role as an alternative energy source. h₂ is considered a clean fuel because it is produced by electrolysis, and the moisture is divided into h₂ and oxygen (O2). It converts to heat or power both efficiently and environmentally friendly. When burning with o2h₂ produces only water without producing any contaminants.
H₂Production is classified according to source and production method, usually by color code:
- Green H₂ – Produced by electrolysis by 100% renewable energy sources such as wind or solar, resulting in a low carbon footprint.
- Blue h₂ – Originated from fossil fuels, but uses carbon capture and storage (CCS) technology to reduce emissions.
- Gray h₂ – Produced from fossil fuels without CCS, emitting significant concentrations (1 ton of H₂ production can release up to 10 tons of carbon) (Dvoynikov et al., 2021).
These categories help the energy industry to distinguish H₂ types. H₂The various methods of production, storage and application are shown in Figure 1.

Figure 1. H₂ production routes, including renewable energy, fossil fuels and nuclear, production of H₂ in power plants, pharmaceutical applications, synthetic fuels or their production in transport, ammonia synthesis, metal production or chemical industry applications (Osman et al.). , 2022).
h₂ is a small and highly diffused molecule that can easily leak during production, storage and distribution, which may reduce its climate benefits. When H₂ is released into the atmosphere, approximately 70%–80% is absorbed by the soil, while the remaining 20%–30% reacts with hydroxyl (OH) radicals. This oxidation process increases the atmospheric concentrations of methane (CH₄), ozone (O₃) and water vapor (H₂O), thereby enhancing radiation forcing.
The key result of H₂ oxidation is the depletion of OH radicals, which in turn extends the lifespan of Ch₄ and increases its atmospheric abundance. (Derwent et al., 2001). Figure 2 illustrates the oxidation process of H₂ in the atmosphere.

Figure 2. Effects of H₂ oxidation in the atmosphere (Ocko & Hamburg, 2022).
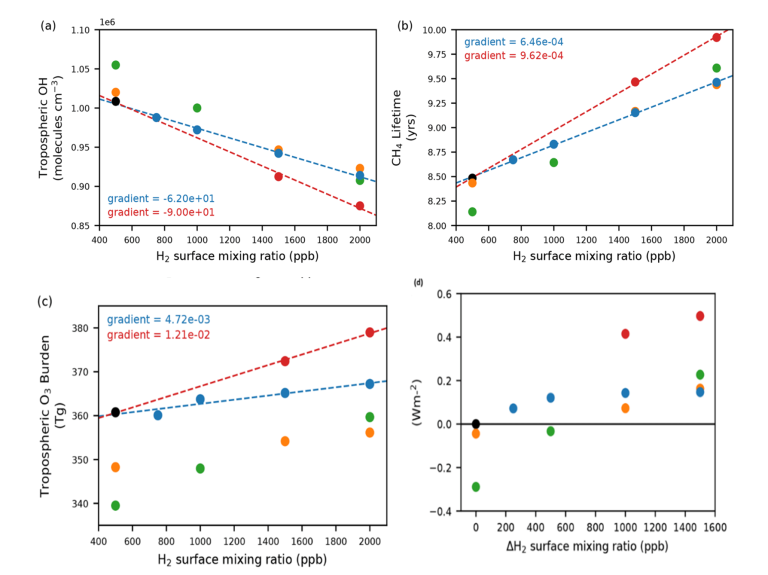
Completed work New Jersey WorryCK, etc. ((2023) use this UKESM Model By increasing h2 The mixing ratio on the surface is from the mixing ratio in 500, 1000, 1500 and 2000 ppb. The results showed that the concentration of OH radicals decreased (Fig. 3A), the increase in CH₄ lifetime (Fig. 3B), and the increase in the concentration of O₃ in the troposphere (Fig. 3C). These findingss Indiathis Elevated concentrations can enhance radiation forcing (Figure 3D).

Figure 3. (a) Large-scale weighted tropospheric mean OH, (b) CH4 lifetime related to OH, (C) Increased ERF in tropospheric O3 and (d) UKESM1. In panels (a) – (d), black circles (basic scene), blue circles (CH4 LBC remains at 2014 grade), red circles (CH4 LBC is adjusted to illustrate changes in CH4 lifespan), orange circles (ozone) changes in precursor emissions). Green circles (emission changes in CH4 and ozone precursors and adjusted CH4 LBC). See NJ Warwick et al. (2023).
Global Warming Potential (GWP) is a key indicator that compares the warming effects of different greenhouse gases against CO₂, usually 100 years (GWP100). Since H₂ is an indirect greenhouse gas, its GWP is affected by the influence on Ch₄, O₃ and stratospheric H₂O. However, the main uncertainty when calculating H₂GWP comes from the soil sink process in different models. In all models, a multimodal study with uniform soil sink of 59 tg yr-1 estimated a GWP100 of H2 of 11.4±2.8 (Sand et al., 2023). Weik et al.'s research. (2023) Using the UKESM1 model with the specified H₂ surface concentration, H₂GWP was estimated to be up to 12±6. Although the warming effect of H₂ is effective, it is relatively short-lived compared to methane. Some of its effects occur within a decade of emissions, but its effects on methane have expanded its climate impact for about a decade (N. Warwick et al., 2022) .
At the University of Reading, I am studying the effect of using the UKESM model on H₂ emissions on atmospheric composition and climate. We have performed multiple 40-year model simulations under current and future H₂ and CH₄ concentration schemes, thereby separating the contribution of effective radiation forcing (ERF) from O₃, aerosol and stratospheric H₂O. Additionally, we decompose each ERF component in clean weather (without aerosol) and transparent (without cloud) conditions.
This method requires the diagnosis and calculation of atmospheric top radiation flux under three conditions (Ghan, 2013): All swans, clean sky (ignoring aerosol scattering and absorption), and clearing clean sky (ignoring cloud and aerosol interaction). Preliminary results show that clouds play a crucial role in regulating radiation forcing by changing the properties of aerosols.
To further explore the complex interactions affecting Ch₄, O₃ and H₂ evolution, we conducted pulse experiments in which 1 month of H₂ emission pulses were introduced globally and in specific regions. These experiments will help quantify the amplitude and timing of changes in H₂, CH₄ and O₃, thereby improving our understanding of the indirect effects of H₂ on atmospheric chemistry and climate.
refer to
Derwent, RG, Collins, WJ, Johnson, CE, & Stevenson, DS (2001). Transient behavior of tropospheric ozone precursors in global 3-D CTM and their indirect greenhouse effects. Climate change,,,,, 49(4), 463–487. https://doi.org/10.1023/a:1010648913655
Dvoynikov, M., Buslaev, G., Kunshin, A., Sidorov, D., Kraslawski, A.. , & Budovskaya, M. (2021). New concept of hydrogen production and storage of resources in the Arctic. https://doi.org/10.3390/Resources100
Ghan, SJ (2013). Technical Note: Estimate the effect of aerosols on cloud radiation forcing. Atmospheric Chemistry and Physics,,,,, 13(19), 9971–9974. https://doi.org/10.5194/acp-13-9971-2013
Ocko, IB, & Hamburg, SP (2022). Climate consequences of hydrogen emissions. Atmospheric Chemistry and Physics,,,,, twenty two(14), 9349–9368. https://doi.org/10.5194/acp-22-9349-2022
Osman, AI, Mehta, N., Elgarahy, AM, Hefny, M., Al-Hinai, A., Al-Muhtaseb, AH, & Rooney, DW (2022). Hydrogen production, storage, utilization and environmental impact: review. exist Environmental Chemistry Letters (Volume 20, Issue 1, pp. 153-188). Springer Science and Business Media Deutschland GmbH. https://doi.org/10.1007/s10311-021-01322-8
Sand, M., Skeie, RB, Sandstad, M., Krishnan, S., Myhre, G., Bryant, H., Derwent, R., Hauglustaine, D., Paulot, F., Prather, M. , & & & D. Stevenson (2023). Multi-model evaluation of the global warming potential of hydrogen. Communication to the Earth and the Environment,,,,, 4(1). https://doi.org/10.1038/s43247-023-00857-8
Warwick, P. Griffiths, J. Keeble, J., Archibald, A., Pyle, J. , & Shine, K. (2022). The atmospheric significance of increasing hydrogen.
Warwick, NJ, Archibald, AT, Griffiths, Pt, Keeble, J., O'Connor, FM, Pyle, Ja, & Shine, KP, KP (2023). The atmospheric composition and climate impact of the future hydrogen economy. Atmospheric Chemistry and Physics,,,,, twenty three(20), 13451–13467. https://doi.org/10.5194/acp-23-13451-2023